毕业论文关键词:多组分串联反应;微波辐射;吖啶衍生物
Microwave-assisted Synthesis of Pyrrole-spiro Acridines with Potential Biological Activity
Abstract: A multi-component domino reaction of 1,3-dicarbonyls with substituted isatins and 2-aminoprop-1-ene-1,1,3-tricarbonitrile was developed under microwave irradiation, efficiently providing a series of acridine derivatives with potential biological activity. This reaction underwent cascades process involved Knoevenagel condensation, Michael addition, intramolecular cyclization, and ring-open of pyrrolidin-2-ones as well as second cyclization, in which the transannulation of isatins was realized. This method has many advantages including short reaction times, simple operation, easy product separation and purification.
Keywords: multi-component domino reaction; microwave heating; acridines
1 前言
1. 1 选题的依据和意义
吖啶类化合物是一类含氮有机杂环化合物,且拥有一个较大的共轭体系,具有很强的刚性平面结构,荧光性很强[1-2],是一类良好的荧光试剂。另外,吖啶可以很好地嵌入到DNA双螺旋中[3],在抗肿瘤[4]、抗菌[5]、抗血吸虫[6]等方面都表现出了很强的生物活性。如,从煤焦油中提炼出来的吖啶黄不仅是常见的染料,还具有较强的抗菌能力。许多天然存在的生物碱中也都存在吖啶骨架,其中具有代表性的有Stellettamine[7]、Cyclodercitin[7-8]和Plakinidines[9](图1),它们都表现出了广泛的生物活性。
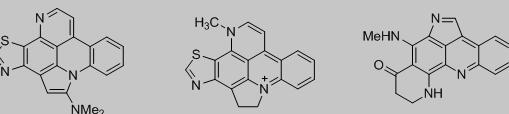
图1 含吖啶骨架的天然生物碱
图2 具有生物活性的吖啶衍生物的结构
近年来,吖啶类药物在抗肿瘤方面的研究取得显著的进展[4,10-11],如图2所示:苯胺吖啶 (m-AMSA, 1),它是进入临床研究的首个全合成吖啶类抗肿瘤药物,能够很好地与DNA以及拓扑异构酶形成复合物,表现出较好的疗效,目前已被用于白血病的治疗[12];DACA (又名XR5000, 2) 具有DNA拓扑异构酶Ⅰ/Ⅱ的双重抑制功能,主要用于非小细胞肺癌和晚期卵巢癌等的治疗,现在已处于临床二期研究阶段[13];BRACO-19 (3) 作为DNA嵌入剂和端粒酶抑制剂,能够很好地抑制癌细胞的生长,其IC50值达到了115 nmol/L[14,15]。
这些化合物的结构和性质都证明了合成新型吖啶类衍生物的重要性,因此,对吖啶衍生物的研究具有非常重要的意义。
1. 2 吖啶类衍生物的合成进展
目前,合成吖啶类化合物的方法,归纳起来主要有以下几种:(1) Bernthsen法,该方法是在ZnC12的催化作用下,使二苯胺与羧酸类化合物发生缩合反应[16]。(2) Unmann法,该方法是利用取代的邻氨基苯甲酸与溴苯或利用取代的邻卤苯甲醛与取代的苯胺进行缩合[17]。(3) Conrad-Limpaeh-knorr法,该方法以β-酮酸酯与芳胺缩合,在浓硫酸的作用下进行环合[18]。(4) Friedländer法,该方法是用邻氨基苯甲醛或邻氨基胡椒醛在酸或碱的条件下,与α-位有活泼亚甲基的醛或酮进行缩合[19]。(5) Pfitzinger法,该方法是利用靛红在碱性条件下生成邻氨基芳酮,再与含有羰基和亚甲基的化合物进行缩合[20]。
王香善等[21]利用芳香醛、5,5-二甲基-1,3-环己二酮及对甲基苯胺在以乙二醇为溶剂,微波辐射下反应生成一系列新的9-芳基-10-对甲苯基多氢吖啶衍生物。该方法产率高,反应所需时间短。