毕业论文关键词:2-氨基-N'-芳基苯甲酰肼、噻吩并吡唑并喹啉、CuI、合成
CuI-Catalyzed Synthesis of Thienopyrazoloquinoline Derivatives
Abstracts: The reaction including 2-amino-N'-phenylbenzohydrazide and 3-bromo-thiophene-2- carbaldehyde is described in this paper to give a series of thienopyrazoloquinoline derivatives in 1,4-dioxane using K2CO3 as base and CuI as catalyst. The structures of products were characterized by HRMS, 1H NMR, and IR spectra. This method has the advantages of excellent yields, convenient processing, and environment friendly.
Keywords: 2-amino-N'-phenylbenzohydrazide, thienopyrazoloquinoline, CuI, synthesis
1. 前言
1.1 选题的依据和意义
喹唑啉衍生物是一类具有良好生物活性的重要的化合物,具有很好的药理和生物活性,越来越受到人们的重视,而2-取代的喹唑啉酮衍生物也被证实具有降压、抗菌和抗癌等的生物活性[1-3]。
1.2 本课题目前的研究状况与水平
Chen等[4]在乙醇中60 °C用靛红酸酐、芳醛及醋酸铵在催化剂Ga(OTf)3的作用下,合成喹唑啉酮衍生物(见Scheme 1)。
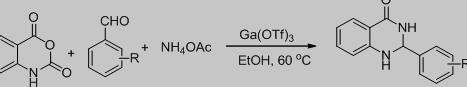
Scheme 1. 2-芳基喹啉酮衍生物的合成
Nanda[5]等以2-苯基-3,1-苯并恶嗪-4(3H)-酮为原料,首先与水合肼回流条件下得到得到3-氨基喹唑啉-4(3H)-酮;后者再和取代的苯甲醛作用合成了3-芳亚胺基喹唑啉-4(3H)-酮衍生物(见Scheme 2),产率较高在70~80 %之间。
Scheme 2. 3-芳亚胺基-2-苯基喹唑啉-4 (3H)-酮衍生物的合成
Narasimhulu[6]等用邻氨基苯甲酸、NH3和原甲酸酯为原料,以La(NO3)3•6H2O或对甲苯磺酸作为催化剂,在乙醇为溶剂条件下三组分合成一系列的喹唑啉酮衍生物(见Scheme 3)。
Scheme 3. 3-取代喹唑啉酮衍生物的合成
Bavetsias[7]通过用过氧化氢脲作为氧化剂,将2-氨基苯甲腈通过两步反应合成了2-甲氧甲基-4(3H)-喹唑啉酮(见Scheme 4)。
Scheme 4. 2-甲氧甲基-4(3H)-喹唑啉酮的合成
Hess等[8]报道了由2-氨基苯甲酸甲酯合成2-氨基-4(3H)-喹唑啉酮的路线。该法通过在过量的氢氧化钠下,温度为130 °C下2-氨基苯甲酸甲酯与过量的胍加压反应5 h,得到2-氨基-4(3H)-喹唑啉酮(见Scheme 5),产率66 %。该合成方法路线较短,反应较为简单,是合成2-氨基-4(3H)-喹唑啉酮较好的方法之一。
Scheme 5. 2-氨基-4(3H)-喹唑啉酮的合成
Anetee等[9]通过以邻氨基苯酰胺为原料的亲核取代反应制备2-乙烯基取代-4(3H)-喹唑啉酮(见Scheme 6),该方法产率高,步骤少,而且所得产物可以作为中间体合成其它的2-取代-4(3H)-喹唑啉酮。